Press Release
Safer Batteries Made With Wood
Nature's 'bio-structure' helps UMD/MSE engineers solve Li metal battery failure problems.
FOR IMMEDIATE RELEASE March 21, 2017
CONTACT:
Martha Heil
(626) 354-5613
mjheil@umd.edu
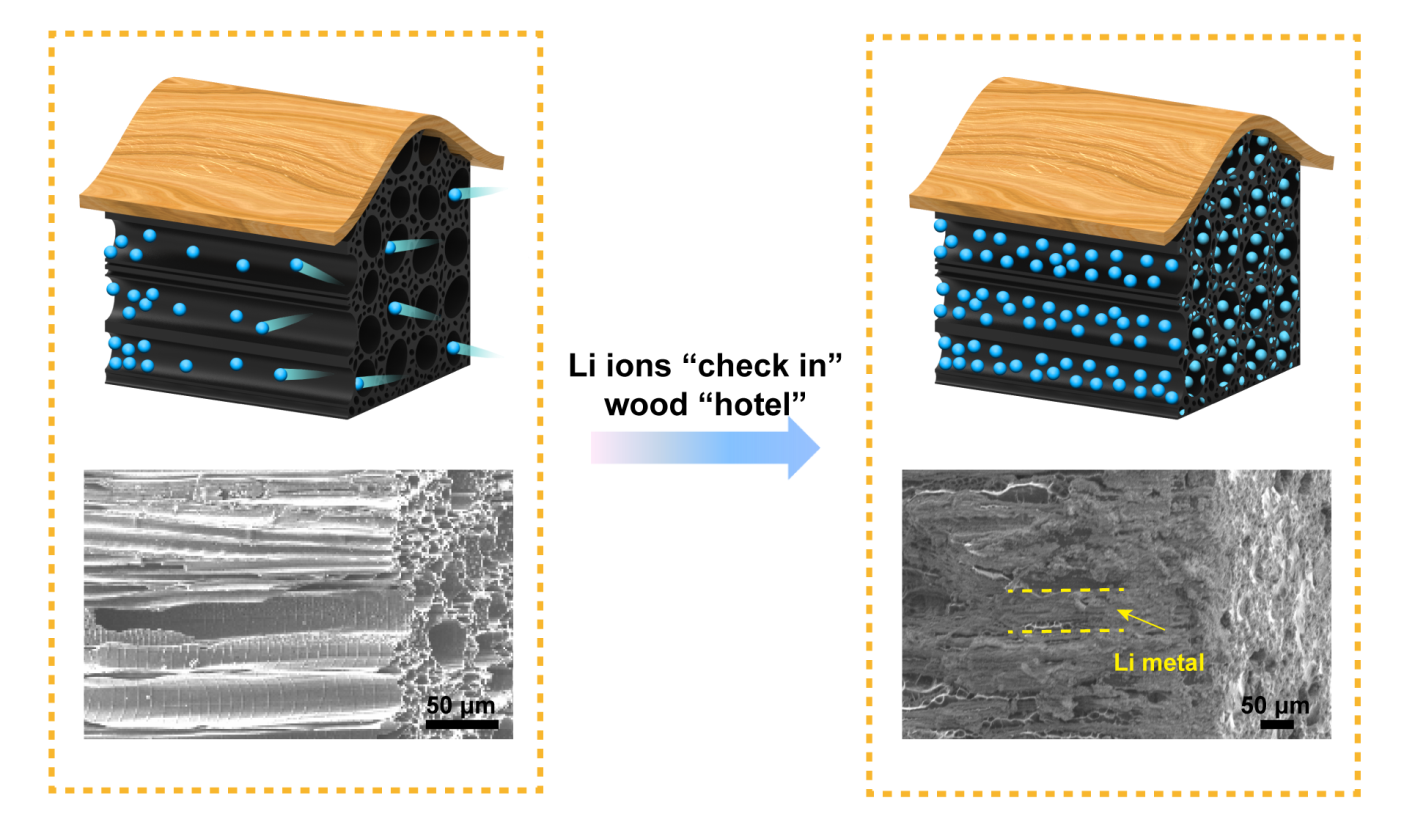
Inspired by the structure of wood, engineers at the University of Maryland have used modified wood as a unique architecture for the negative electrode of a lithium (Li) metal battery, seeking to prevent some of the key factors that lead to battery failure.
Lithium ion shuttling in rechargeable batteries provides energy to power your phone, laptop, or even just a light bulb. When the battery is charged, the lithium metal expands; and when it is discharged, the lithium metal deflates. This rapid change in size can lead to an undesirable side-effect, branch-like growths of lithium on the surface of the lithium metal. The damage builds up over time and can pose safety hazards like overheating or fire. This novel design for a safer Li metal battery created by Ying Zhang, a UMD Ph.D. student in the department of materials science and engineering, can be used to boost the energy density of a battery; thereby increasing the power available for portable electronics and electric vehicles, while reducing the risk of the battery overheating.
In this new type of battery, instead of storing lithium particles (ions) in a metal block, the engineer's store lithium in the natural channels of carbonized wood, channels that were once used to carry water and nutrients.
The wood acts as a “hotel” to provide lots of rooms (channels) to accommodate many guests (lithium metal). As the lithium metal “guests” enter this wood hotel, it can accommodate them all, storing them comfortably and securely in each room while maintaining the wood’s rigid exterior structure. The number of lithium particles (guests) can increase and decrease within each room, but the overall structure will not be damaged or collapse.
“This is part of our ongoing research to use natural materials to improve batteries,” said group leader Liangbing Hu, an Associate Professor in the Department of Materials Science and Engineering and a member of the University’s Energy Research Center (UMERC). “Using nature’s bio-structure, we can find inspiration to create new ways of storing energy, and we can use renewable materials, too.”
This research was published in the Proceedings of the National Academy of Sciences on March 20, 2017, in a paper entitled, "High Capacity, Low Tortuosity and Channel-Guided Lithium Metal Anode."
Related article: http://www.mse.umd.edu/news/news_story.php?id=10377
About the A. James Clark School of Engineering
The University of Maryland’s A. James Clark School of Engineering is a premier program, ranked among the top 20 in the world. Located just a few miles from Washington, D.C., the Clark School is at the center of a constellation of high-tech companies and federal laboratories, offering students and faculty access to unique professional opportunities.
Our broad spectrum of academic programs, including the world’s only accredited undergraduate fire protection engineering program, is complemented by a vibrant entrepreneurial ecosystem, early hands-on educational experiences, and participation in national and international competitions.
The Clark School is leading research advancements in aerospace, bioengineering, robotics, nanotechnology, disaster resilience, energy and sustainability, and cybersecurity. From the universal product code to satellite radio, SMS text messaging to the implantable insulin pump, our students, faculty, and alumni are engineering life-changing innovations for millions. Learn more at www.eng.umd.edu.