Institutional Review Board (IRB)
Institutional Review Board ∙ irb@umd.edu ∙ 301-405-4212 ∙ 1204 Marie Mount Hall ∙ Hours: 8:30 AM - 4:30 PM
Welcome to the Institutional Review Board (IRB) Office
- What is an IRB?
- Review Paths
- IRB Composition
- Federalwide Assurance
- Standard Operating Procedures and Policies
- Virtual Appointments with the IRB Office
- Time to IRB Review
- Full Board Meeting Dates and Deadlines
What is an IRB?
An Institutional Review Board (IRB) is a committee that performs ethical review of proposed research to help assure the protection of the rights and welfare of human participants. The IRB approves the initiation of and conducts periodic reviews of research involving human participants. Investigators also share the responsibility for protecting human participants.
To submit an application please visit IRBNet.
If you do not have an account, go to IRBNet, then click “New User Registration” in the upper right hand corner and follow the instructions. Your IRBNet account is NOT linked to your UMD ID.
For more information on how to submit via IRBNet, please click here.
Review Paths
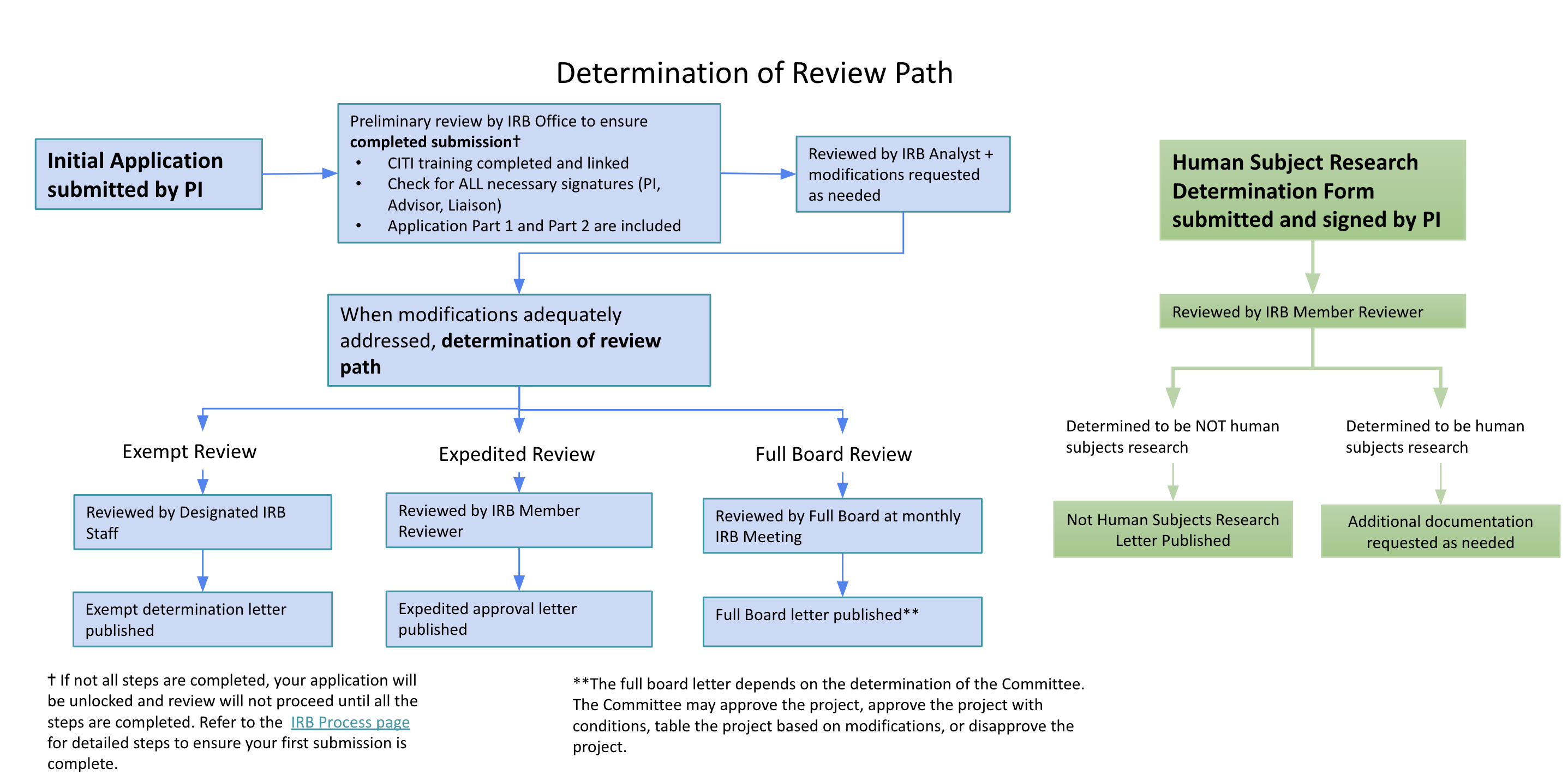
Human Subject Research Determination
If you are unsure if your project requires IRB review and approval, please download, complete, and submit the HSRD Form through IRBNet. You will receive an official determination confirming no IRB Approval is needed or additional information will be requested.
Exempt Review
To receive an Exempt Determination from the IRB, a protocol must fall into one or more of eight (8) federally-defined exempt categories. Examples of Exempt research are: anonymous surveys/interviews, passive observation of public behavior without collection of identifiable information, retrospective chart/record/data reviews, analysis of discarded pathological specimens without identifiers, etc.
Expedited Review
To qualify for an Expedited Review, research should fall into one or more of nine (9) federally-defined Expedited categories and present no greater than minimal risk to participants.
Full IRB Review
Protocols which do not meet either Exempt or Expedited review criteria will be added to the next available agenda for review at the fully convened IRB Meeting. Research activities presenting greater than minimal risk or transactions increasing the potential risk, will always be referred to the full convened IRB Meeting for review.
Submission Deadlines and Meeting Dates are available in the table at the bottom of this page.
IRB Composition
The IRB is a review committee consisting of faculty, students, staff, and outside members that has been established to help protect the rights and welfare of human participants participating in research. It is established to evaluate the ethical implications and conduct a risk-benefit analysis of research involving human participants through the application of 45CFR46 (Code of Federal Regulations governing human subject research).
For additional information regarding the review committee, the Office for Human Research Protections' (OHRP) provides the webinars listed below:
- IRB Review Criteria
- Quorum and Voting in IRB Meetings
- Membership Requirements for Institutional Review Boards (IRB)
Federalwide Assurance
FWA: 00005856
IRB Registration: IRB00000474
IRB Organization: 0000281
To check the expiration dates for these Assurances, please refer to the Department of Health and Human Services website for the Office for Human Research Protections(OHRP) database.
The University of Maryland College Park Federal Wide Assurance and the IRB registration are listed under "U of Maryland Coll Park Campus."
Standard Operating Procedures and Policies
This resource provides a detailed outline and thorough description of the Standard Operating Procedures that are specific to the UMD Office of IRB Operations:
IRB Office - Standard Operating Procedures (PDF)
This is the overarching University Maryland System Policy on Human Subjects of Research:
IV-2.10- UNIVERSITY OF MARYLAND SYSTEM POLICY ON HUMAN SUBJECTS OF RESEARCH
Virtual Appointments with the IRB Office
The IRB Office offers virtual appointments. Virtual appointments are designed to provide UMD researchers with an opportunity to meet with an IRB Analyst to provide general IRB guidance, answer questions regarding IRB processes, and to offer technical assistance with IRBNet.
Each appointment will be limited to 15 - 30 minutes.
To register for a virtual IRB appointment, please complete this form in its entirety. You must complete this form using your UMD-affiliated email address.
Please Note: The IRB staff will not provide any IRB determinations. In order to receive an official IRB determination, please submit an IRB application through IRBNet.
If your question requires immediate attention, please email irb@umd.edu.
Time to IRB Review
Estimates for time to IRB review for HSRD submissions and studies undergoing exempt and expedited review are below. Most studies at UMCP meet the definition of minimal risk and thus receive exempt or expedited determinations - these studies are reviewed on a continuous basis and are generally NOT subject to the Full Board deadlines below.
| Application Type | Time to IRB Review* |
|---|---|
| Initial Application (NOT Full Board) | 2-3 weeks from completed submission |
| Amendment | 1-2 weeks from completed submission |
| Human Subject Research Determination (HSRD) | 1 week from completed submission |
*Time to IRB review is the estimated time it takes from the date of completed submission to the time when modifications are requested by IRB staff. This is not the same as the time to approval.
*Note that these are estimates and times may vary based upon timing in the semester and current volume of submissions. The busiest times of year at the IRB are: mid-Fall semester, prior to Thanksgiving break, prior to Winter Break, mid-Spring semester, and prior to Spring Break. Please also note that the IRB Office is closed when the University is closed.
In general, completed submissions are reviewed in the order in which they are received. Investigators with urgent requests requiring immediate review should contact irb@umd.edu and briefly describe the specific circumstances. We will do our best to accommodate your request.
Avoiding Delays in Review
- Consult the IRB Process page to ensure you have a completed submission.
- Review the IRB Submission Tips (PDF) for required elements and suggestions for preparing materials.
- Respond to modifications promptly. The faster you are able to address modifications requested by the IRB staff, the faster the project will be moved forward to the final reviewer. In other words, projects with ongoing review are prioritized over new submissions.
Full Board Meeting Dates and Deadlines
Per Federal regulations and institutional policy the Full Board reviews protocols where one or more of the following are true of the research: (1) it involves greater than minimal risk to human subjects, (2) it does not meet the criteria for one of the categories of expedited review, and/or (3) it has been referred to the committee by an IRB Member.
Regardless of risk level, a project may require full board review when the research involves:
- Vulnerable populations (including prisoners)
- Sensitive topics, including illegal activity
- Complex research design/methods requiring the expertise of multiple board members to evaluate
| IRB Meeting Date | Submission Deadline |
|---|---|
| November 14, 2024 | Occtober 27, 2024 |
| December 12, 2024 | November 24, 2024 |
| January 16, 2025 | December 29, 2024 |
| February 13, 2025 | January 26, 2025 |
| March 13, 2025 | February 23, 2025 |
| April 10, 2025 | March 23, 2025 |
| May 8, 2025 | April 20, 2025 |
| June 12, 2025 | May 25, 2025 |
| July 10, 2025 | June 22, 2025 |
| August 7, 2025 | July 20, 2025 |
| September 11, 2025 | August 24, 2025 |
| October 9, 2025 | September 21, 2025 |
| November 13, 2025 | October 26, 2025 |
| December 11, 2025 | November 23, 2025 |
| January 15, 2026 | December 28, 2025 |

