Press Release
John Cumings and Jasper Drisko Published in Nature Communications
Small defects can make whole materials frustrated.
FOR IMMEDIATE RELEASE January 13, 2017
CONTACT:
Katie Doyle
301 405 0379
khollan3@umd.edu
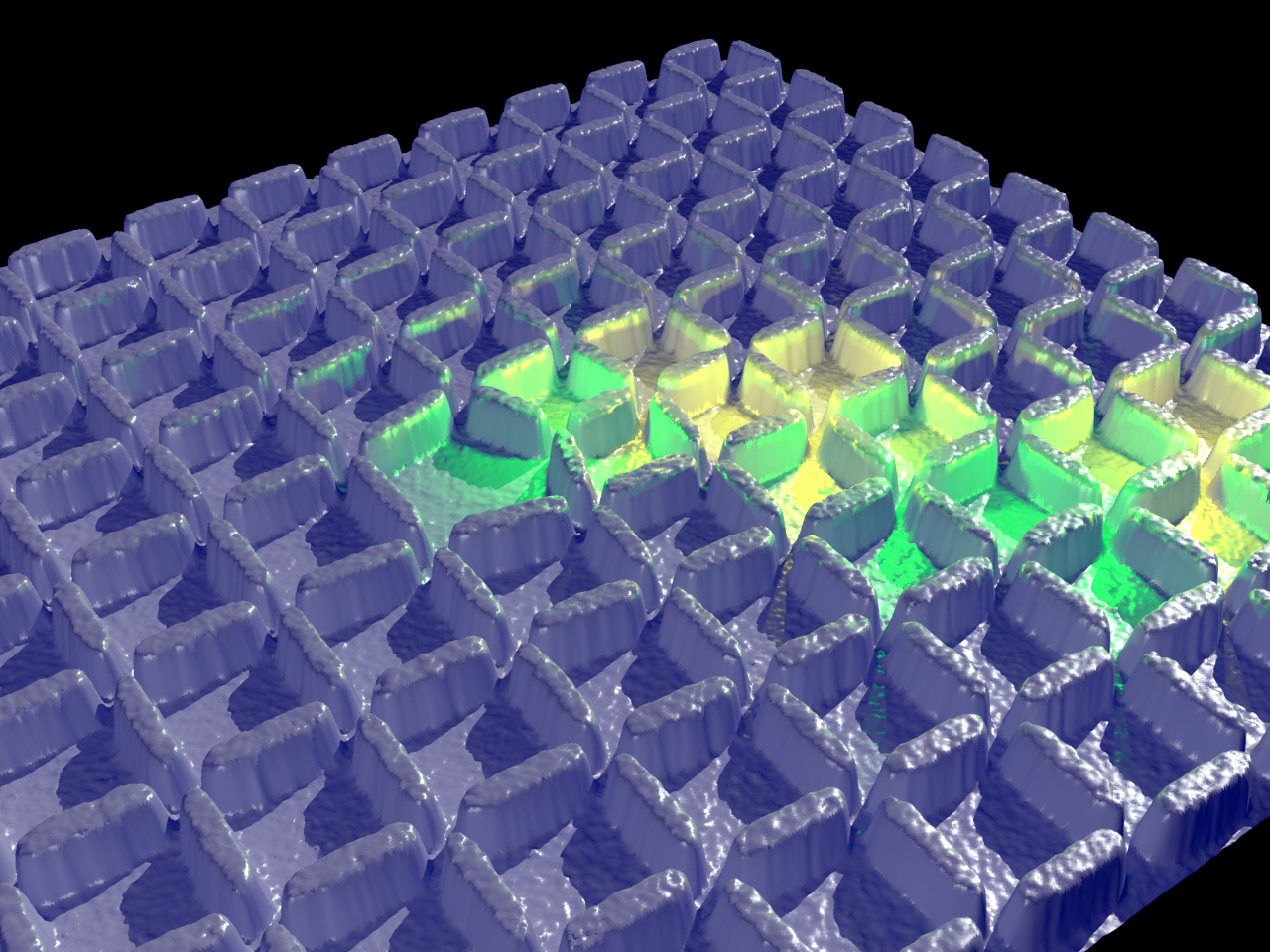
Artist's rendering of artificial spin ice containing a topological defect. The defect causes ‘frustration,’ which produces a kink in the ground state, illuminated above in green and yellow.
COLLEGE PARK, Md. | A ground state -- the lowest possible level of energy that an atom or a group of atoms can achieve -- is a property of all systems of materials. Some materials, however, don't have an established ground state that scientists can reliably identify. Researchers at the University of Maryland think they know at least one reason this could be happening: Tiny defects causing bigger ripples.
Each atom in a material has spin, which is a bit like its magnetic orientation. Atoms in a material influence one another through their spin and are happiest when neighboring atoms have opposite spins, just as magnets’ opposite poles attract. When all atoms are at peace with their neighbors – when they’re all at equilibrium – they’ve achieved their ground state. But what if the material forms a triangle – what does the third spin do? It would, at the very least, throw a few nearby atoms off balance as they adjust to the oddball’s spin. When defects create this type of imbalance, scientists call them topological defects, and they are almost inevitable in most crystalline materials.
Materials scientist John Cumings and his student, physicist and UMD alumnus Jasper Drisko, deliberately made a defect in a model of a material and found that a small change made a big difference across the whole material.
“No material is perfect, but sometimes scientists assume that a small defect doesn’t have an overall effect,” said Cumings. “Our work shows that a small defect can alter the ground state across the whole material.”
They used a magnet-based model of a type of material called spin ice, which is easy to manipulate. Each tiny magnet is only a few hundred nanometers long – one thousand times less than the thickness of a sheet of paper. Drisko’s previous work demonstrated that the best material to use in the model is an iron-palladium alloy, which allows the magnets to flip freely and find their happiest, ground state arrangements. Their tiny size means that the magnet can only orient in one direction.
The pristine structure of spin ice is a nice neat square, with pairs of atoms spinning happily together. The scientists replicated those squares in their magnet model, but with one teeny change: one square in the middle of the material was changed to a pentagon shape. This confused – or, more technically, “frustrated” -- the magnets, which then showed a path of mis-turned magnets all the way to the edge of their sample. Worse, it wasn’t as though the same path appeared every time – creating a defect made other paths in all different directions, or even circles. In any case, the material, because it was disturbed, couldn't reach its ideal consistent ground state.
A paper based on the study, “Topological frustration of artificial spin ice,” http://dx.doi.org/10.1038/ncomms14009 published online January 13, 2017, in the peer-reviewed journal Nature Communications.
“This work shows the power of intentional design in artificial spin ice. Because the nanostructure can be designed with a defect included, and then probed in detail, artificial spin ice provides a unique window into how nature accommodates defects in regular structures,” says Peter Schiffer of the University of Illinois at Urbana-Champaign.
“Our work has broad implications for other materials,” said first author Drisko. “Other systems beyond the spin ices could be frustrated or complicated by topological defects and this is something that scientists need to consider more often in theoretical and experimental observations, when they are considering ground states.”
A co-author on the paper was a high school student at the time the work was done. Thomas Marsh, a student at St Albans High School when he helped with the data analysis, now attends Hamilton College.
The work was supported by NSF CAREER.
About the A. James Clark School of Engineering
The University of Maryland’s A. James Clark School of Engineering is a premier program, ranked among the top 20 in the world. Located just a few miles from Washington, D.C., the Clark School is at the center of a constellation of high-tech companies and federal laboratories, offering students and faculty access to unique professional opportunities.
Our broad spectrum of academic programs, including the world’s only accredited undergraduate fire protection engineering program, is complemented by a vibrant entrepreneurial ecosystem, early hands-on educational experiences, and participation in national and international competitions.
The Clark School is leading research advancements in aerospace, bioengineering, robotics, nanotechnology, disaster resilience, energy and sustainability, and cybersecurity. From the universal product code to satellite radio, SMS text messaging to the implantable insulin pump, our students, faculty, and alumni are engineering life-changing innovations for millions. Learn more at www.eng.umd.edu.