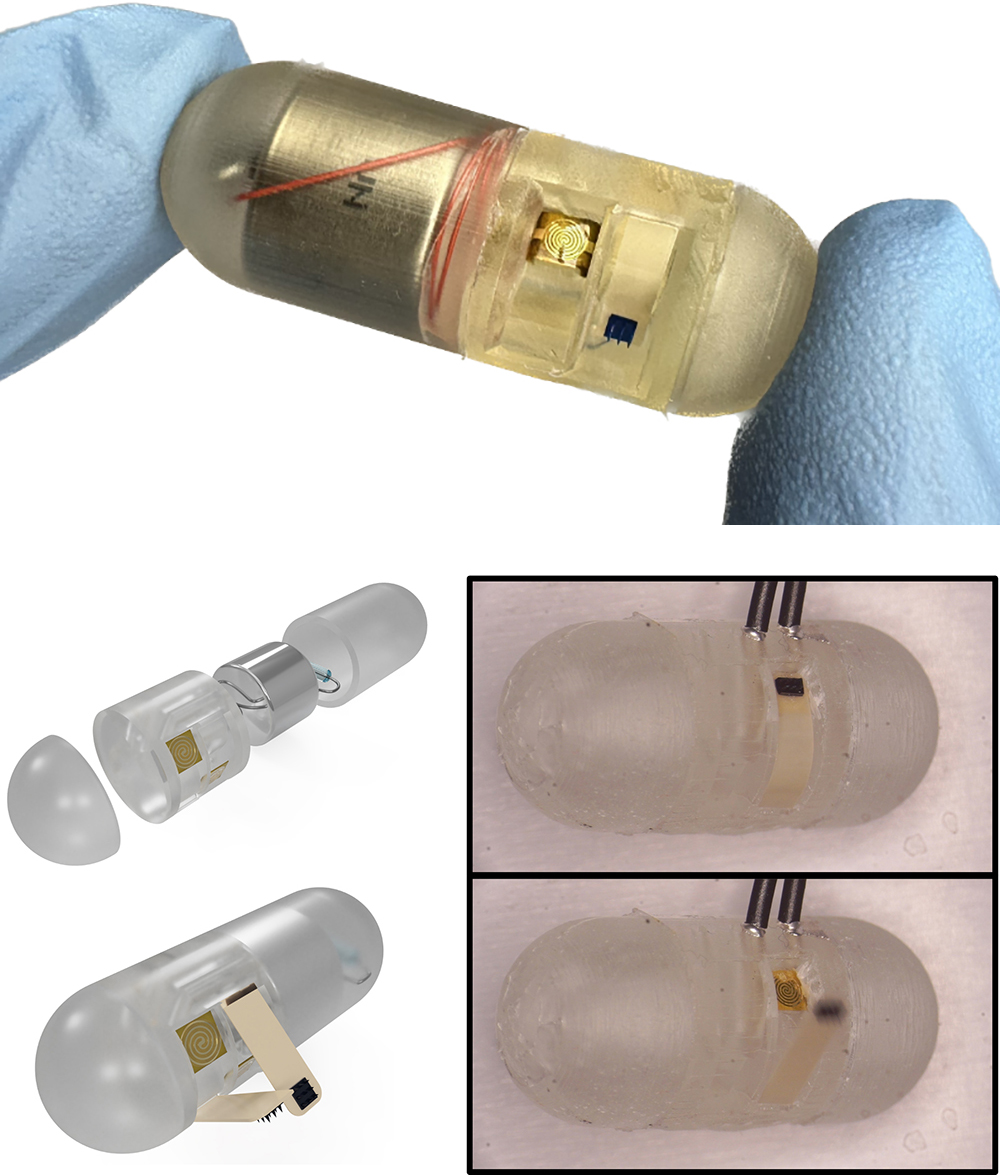
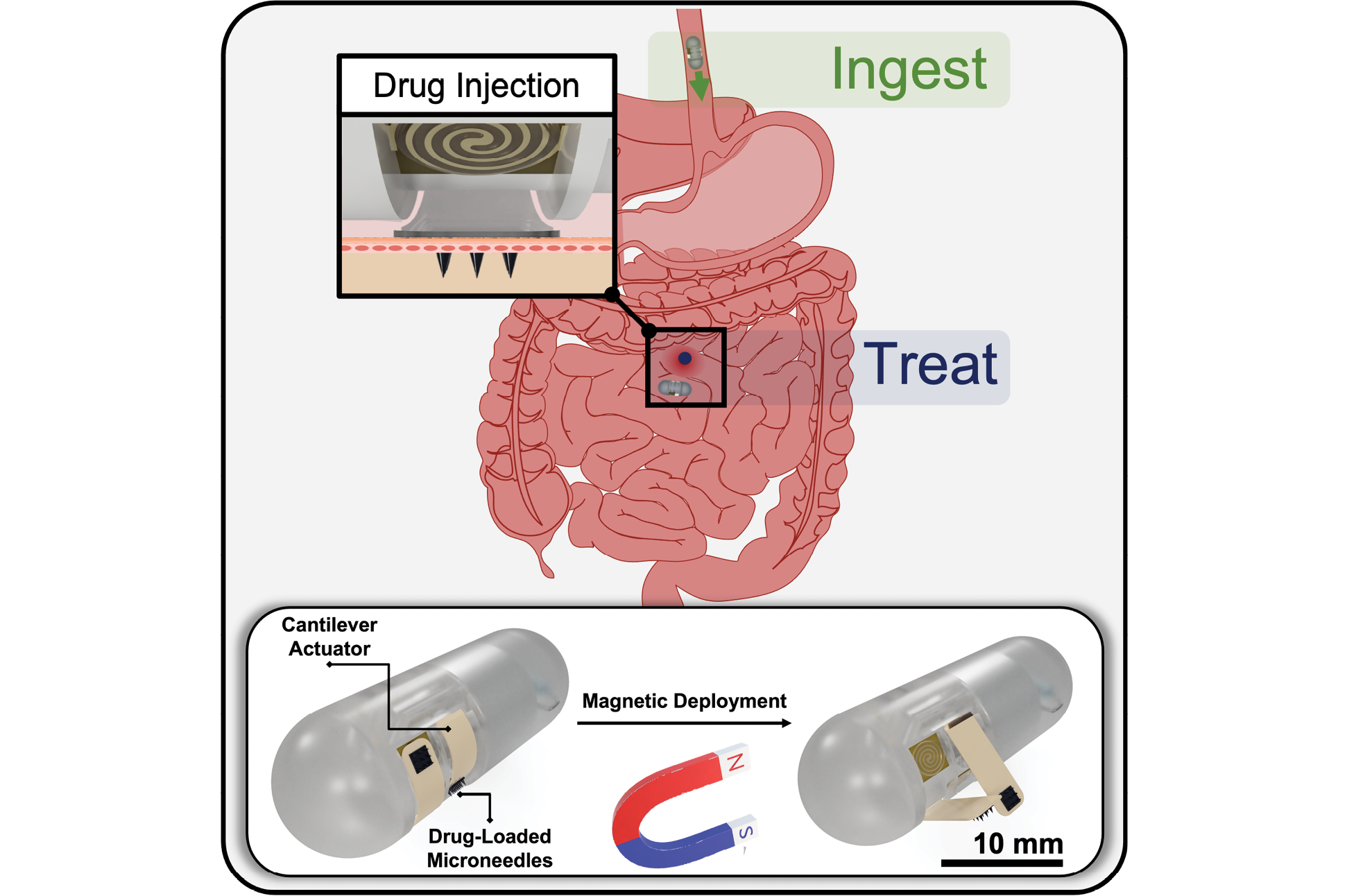
News Story
Huang, Stroka Labs Collaborate to Advance Understanding of Blood-Brain Barrier
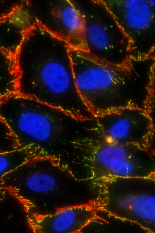
The image shows tight junctions between human brain microvascular endothelial cells being opened by photodynamic priming.
Despite centuries of advancements in the study of medicine and design of biotherapeutics, the human body itself poses one of the biggest challenges to targeting disease treatments to the central nervous system: the blood-brain barrier.
This highly selective, semi-permeable barrier is made up of endothelial cells that line the blood vessels in the brain in order to protect the brain from foreign substances that might be circulating in the blood – all while allowing essential nutrients to reach the brain. When it functions correctly, the blood-brain barrier is an ingenious system that prevents “bad actors” like viruses, parasites, or bacteria from making their way from the bloodstream to the brain, where they could inflict lethal damage. The problem is that, at times, this system is a little too effective as it also blocks potentially lifesaving drugs from reaching key parts of the body via the central nervous system, such as for the treatment of brain or spinal cord tumors or injuries or diseases such as meningitis, multiple sclerosis, or encephalitis.
Recognizing these challenges, Fischell Department of Bioengineering (BIOE) Assistant Professors Huang Chiao Huang and Kimberly Stroka, along with members of their respective labs, joined forces to explore how a specialized photochemistry-based technique could be used to control or perhaps even change the permeability of the blood-brain barrier.
The selective permeability of the brain endothelial cells is controlled by what are known as tight junction proteins. Tight junctions serve as fusion points between cells, and are made up of numerous proteins that join together to form a barrier to fluid. Disrupted junctions are linked to several neurodegenerative diseases such as Alzheimer’s disease and multiple sclerosis – but scientists don’t yet fully know what causes these disruptions. Understanding the key players driving junction stability could therefore hold significant promise for therapeutic discovery. Even more, understanding how cell-cell junctions operate in brain endothelial cells could improve drug delivery into the brain, which is often limited by an intact blood-brain barrier.
To shed light on how and why the blood-brain barrier functions the way it does, the research team used a Python-based program developed by Stroka’s Cell and Microenvironment Engineering Lab. The Junction Analyzer Program (JAnaP) provides quantitative metrics that describe the integrity of cell junction proteins in response to different biochemical and physical cues. By tapping JAnaP to observe cell-cell junctions under different conditions, Stroka, Huang, and members of their respective labs investigated how different factors drive barrier permeability.
“It’s important that as we explore new therapeutic approaches to deliver drugs across the blood-brain barrier, we also understand how and where those drugs are getting across,” Stroka said. “Traditionally, when people assess blood-brain barrier integrity, they perform assays that provide a single measurement that is supposed to be representative of the whole sample. However, the cell-cell junction integrity can vary across different regions of the blood-brain barrier, so just having one number as a measure of barrier integrity won’t tell us anything about what is going on at a more local level, such as where the therapeutic might be getting across. Our JAnaP now allows us to determine the relationship between cell-cell junction integrity and local permeability in a specific spot of the blood-brain barrier.”
To take things a step further, Huang and members of his Optical Therapeutics and Nanotechnology Lab put their expertise to the test. The group worked with Stroka’s lab to apply photodynamic priming – a technique that involves light activation of molecules known as photosensitizers to photochemically alter nearby molecules without killing any cells. In doing so, the collaborative research team demonstrated that photodynamic priming could be used to alter the blood-brain barrier’s permeability without causing harm to the brain endothelial cells that comprise the barrier.
“We're encouraged by the results which suggest it may be possible to open this barrier to deliver drugs in the right location, at the right time and for the right duration, while ensuring healthy brain function,” said Huang. “We look forward to more opportunities to apply this unique approach for a number of brain disorders.”
The work put forth by Stroka, Huang, and their teams not only advances understanding of the conditions that affect the blood-brain barrier’s integrity, but it also points to potential techniques to enhance drug transport across the barrier for the treatment of disease.The collaborative efforts of Stroka, Huang, and their lab groups were highlighted in Fluids and Barriers of the CNSand IEEE Journal of Selected Topics in Quantum Electronics.
BIOE alumna Kelsey (Gray) Goodfriend (Ph.D. ‘19) served as first author of the Fluids and Barriers of the CNS paper titled “Quantitatively relating brain endothelial cell-cell junction phenotype to global and local barrier properties under varied culture conditions via the Junction Analyzer Program.” BIOE senior Jae W. Jung, and Ph.D. student Collin Inglut contributed to the paper along with Huang and Stroka (corresponding author).
Inglut served as first author on the IEEE Journal of Selected Topics in Quantum Electronics paper titled “Photodynamic Priming Modulates Endothelial Cell–Cell Junction Phenotype for Light-Activated Remote Control of Drug Delivery.” (Gray) Goodfriend, Stroka, and Huang (corresponding author) also contributed to the paper, along with BIOE Ph.D. student Shruti Vig, undergraduate student Jillian Stabile, and University of Maryland School of Medicine Associate Professor Yuji Zhang.
Published January 12, 2021